Introduction
Articular cartilage is an avascular, hypocellular tissue with dense collagen fibrils and a protein matrix that provides a low-friction and highly durable wear resistant surface. The complex composition of hyaline cartilage is maintained by chondrocytes residing in the cartilage matrix which contains 90-95% Type II collagen. The collagen fibrils and matrix proteins together form a unique tissue that regulates water content (75% of hyaline cartilage) lubricates, and provides tensile strength to resist mechanical wear.
Following injury or degeneration, the avascular nature and relative low cell count of articular cartilage prevent a repair response that restores the normal architecture of cartilage.
A retrospective study on 25.124 knee arthroscopies showed evidence of chondral/cartilage defects in 15.074 knees (60%), up to 67% were classified as localized and focal defects1.
Since cartilage defects have a poor intrinsic healing capacity, left untreated they can lead to: joint degeneration, chronic pain and disability.
NanoFx: next generation of bone marrow stimulation
Attracting precursor cells into the defect site of cartilage lesions through bone marrow stimulation has been a successful treatment option in cartilage repair. Since the late 1980s, microfracture/drilling has been developed into the procedure of choice due to its low-cost nature, relative low morbidity, and encouraging results as first line treatment for small cartilage defects, especially in young and active patients2,3,4.
Recent insight into the limitations of microfracture and drilling has opened-up new treatment pathways for mesenchymal stem cell stimulation5,6. As an alternative to the shallow 2 mm wide, 3 mm deep microfracture channels, Nanofracture (NanoFx) was introduced in 20136.
When compared to microfracture, this technique provides deeper, consistent, bone marrow access (9 mm deep, 1 mm wide).
Drill-free bone perforations optimise trabecular bone venting and avoid channel closure from K-wire drilling caused by the bone slurry deposits that clog the trabecular tributaries. Chen et al. demonstrated that deeper marrow access leads to an improved biological response and better tissue quality7,8.
The sharp disposable needle tip provides better control during placement of the perforations and avoids slippage and damage to surrounding tissues.
Microfracture

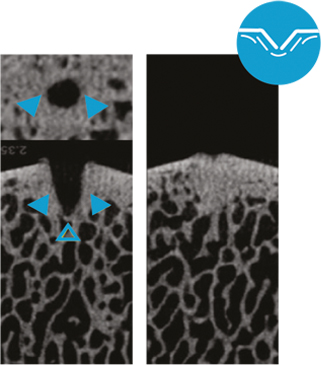
Microfracture: Trabecular wall thickness and density increased by apparent bone compression; limited trabecular channel access; channel borders with non-anatomic regularity; microfracture channel margins: Dense, compressed bone deposit (right)10.
Nanofracture (NanoFx)

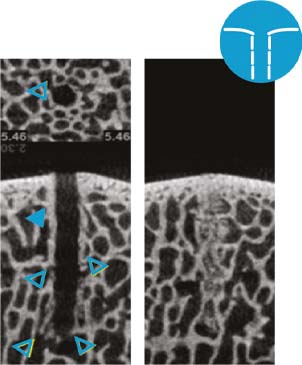
Nanofracture: Trabecular wall thickness and density appears normal; large number of open trabecular channels; anatomic irregularity of trabecular channel borders intact; nanofracture channel margins: course and fragmented trabecular bone deposits (right)10.
K-Wire drilling

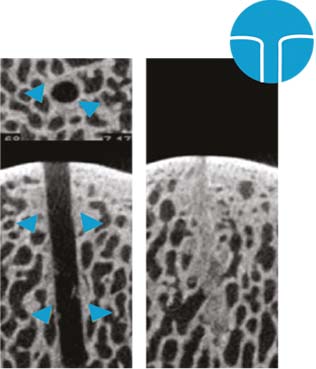
1 mm K-Wire: Trabecular wall thickness and density close to normal; limited trabecular channel access; trabecular channel borders with non-anatomic regularity; K-wire channel margins: pulverized and dense osseous deposits (right)10.
![]() closed trabecular channels, microCT comparison: Axial (top) Sagittal (bottom)
closed trabecular channels, microCT comparison: Axial (top) Sagittal (bottom)
![]() open trabecular channels
open trabecular channels
Summarizing, Nanofracture compared to other bone marrow stimulation techniques results in:
- Diminished areas of destruction, sclerosis, and thickening in regions adjacent to the defect, thereby limiting the amount of perimeter compaction9.
- A subchondral bone needling procedure that reaches to a standardized depth of 9mm deep at a width of 1mm results in thin, fragmented cancellous bone channels without rotational heat generation.
- Superior bone marrow access with multiple trabecular access channels extending 9mm into subchondral bone. Deeper subchondral bone stimulation yietlds better cartilage stimulation, higher collagen Type II content and less Type I.
- Widuchowski W, Widuchowski J, Trsaska T. 2007. Articular cartilage defects: study of 25,124 knee arthroscopies. Knee 14:177–182
- Kreuz PC, Steinwachs MR, Erggelet C, Krause SJ, Konrad G, Uhl M, Südkamp N. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthritis Cartilage. 2006 Nov;14(11):1119-25.
- Mithoefer K, Williams RJ 3rd, Warren RF, Potter HG, Spock CR, Jones EC, Wickiewicz TL, Marx RG. The microfracture technique for the treatment of articular cartilage lesions in tthe knee. A prospective cohort study. J Bone Joint Surg Am. 2005 Sep;87(9):1911-20
- Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003 May-Jun;19(5):477-84.
- Fortier LA, Cole BJ, McIlwraith CW. Science and animal models of marrow stimulation for cartilage repair. J Knee Surg 2012;25:3-8.
- Fortier LA, Cole BJ, McIlwraith CW. Science and animal models of marrow stimulation for cartilage repair. J Knee Surg 2012;25:3-8.t
- Chen H, Sun J, Hoemann CD, et al. Drilling and microfracture lead to different bone structure and necrosis during bone-marrow stimulation for cartilage repair. J Orthop Res. 2009 Nov;27(11):1432-8.
- Chen H, Hoemann CD, et al. Depth of subchondral perforation influences the outcome of bone marrow stimulation cartilage repair. J Orthop Res. 2011 Aug;29(8):1178-84.
- Gianakos et al., The Effect of Different Bone Marrow Stimulation Techniques on Human Talar Subchondral Bone: A Micro eComputed Tomography Evaluation Arthroscopy: The Journal of Arthroscopic and Related Surgery, Vol -, No - (Month), 2016: pp 1-8
- Behrens et al., Bone Marrow Access in Cartilage Repair: Comparison of Microfracture, Nanofracture, K-wire, and Drill in the Adult Ovine Model., e-Poster: P87 Congress: ICRS 2013
